Bioreactors: Essential elements for industrialization
In a world where sustainability is vital for nearly every operation, biotechnological production takes greater significance. For this reason, the use of bioreactors becomes critical for products with scientific backing.
Medicines, biofertilizers, or enzymes used across various industries are the result of metabolic processes occurring within these systems. Below we’ll provide a brief overview of what bioreactors are, how they work, and how scaled up from the laboratory to an industrial level, offering a broader context in the pursuit of sustainable and effective large-scale solutions.
What is a bioreactor?
A bioreactor is a closed vessel that helps cell development under aseptic conditions. It controls variables such as temperature, pressure, pH, and homogeneous on media culture and buffer solutions, as well as the gas distribution.
How does a bioreactor work?
Controlling biological processes, to keep in the right condition for cellular proliferation. Some of the main components of a bioreactor design serve to be considered are as following:
- Vessel: holds the culture medium, buffer solutions, and working cells in a sterile environment to promote cell growth under aseptic conditions. It is typically made of stainless steel.
- Agitation: keeps nutrients in constant motion for uniform distribution, enhancing oxygen transfer and/or other gases that support cell development.
- Temperature control: thermal regulation systems support the ideal range for biological activity, given the sensitivity of the working cells to specific variations.
- Sensors: measure and keep the proper physicochemical conditions of the medium to ensure best cell growth.
- Waste removal: as cells multiply, waste substances that can be toxic are produced. This requires a continuous flow of in and output, such as in continuous culture systems.
Industrial-scale bioreactors are larger, often exceeding volumes of 1 liter. They incorporate advanced technologies to check and control critical parameters.
Types of bioreactors
The market offers a variety of bioreactors, classified based on phases, mixing levels, or mode of operation. Here, we will focus on the third classification.
Batch bioreactors
As the name suggests, these are designed for batch production. Microorganisms or cells are added at the beginning and are not removed until the desired product is obtained. They are recommended for:
- Content instability.
- Small to medium-scale production.
- Low volume or special products.
Based in user experience, batch bioreactors are best suited for:
- High-purity requirements: Certain medication or specialized enzymes receive help from strict control and minimal contamination.
- Sensitive products: if microorganisms are prone to environmental changes, these reactors ensure a stable environment.
- Avoiding by-products: since the reactor is not replenished, by-product accumulation is minimized, preserving product quality.
Industrial scaling of batch bioreactors is often limited because their operation requires downtime for cleaning between cycles, leading to higher maintenance costs.
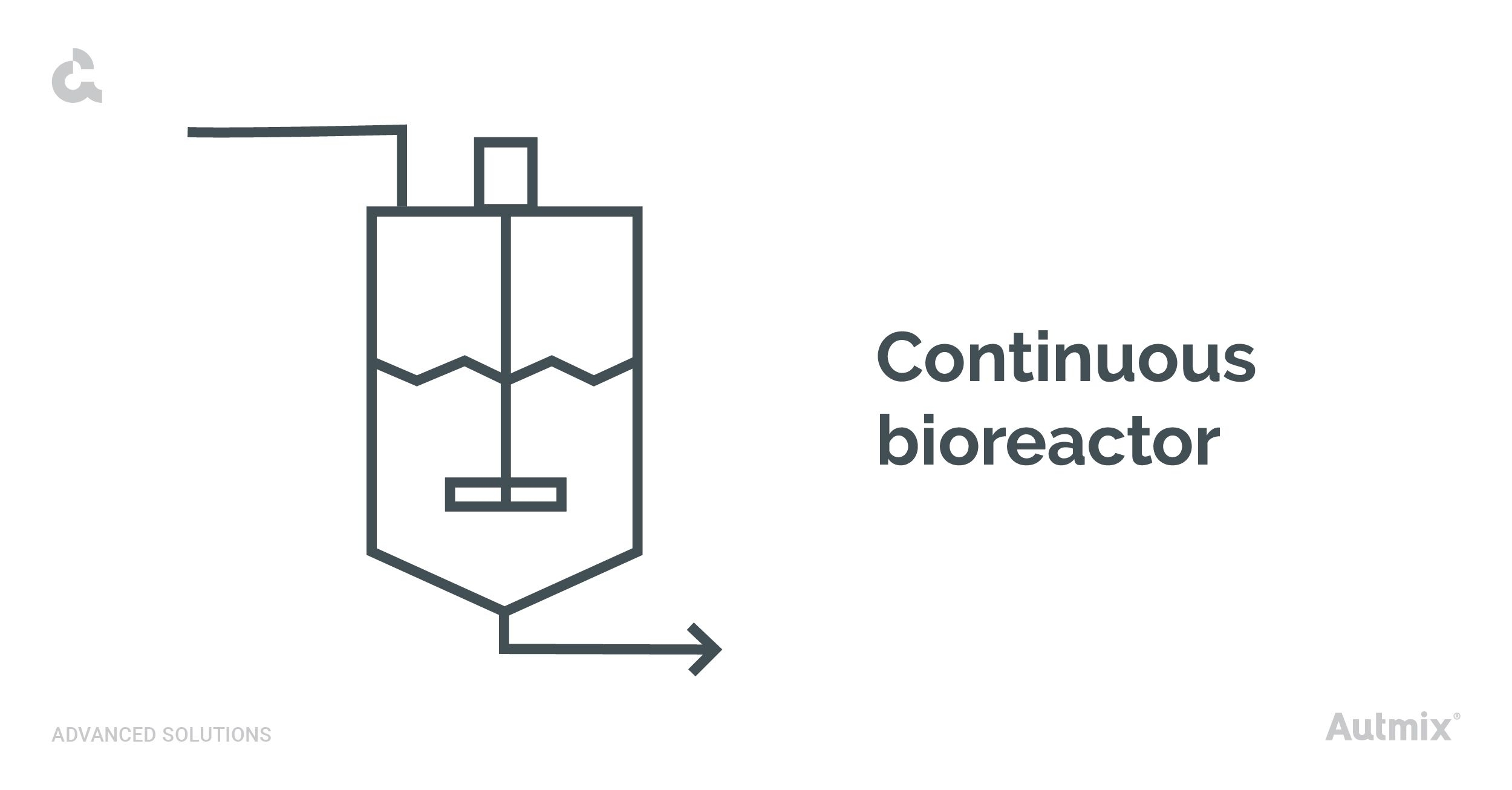
Continuous bioreactors
Continuous bioreactors are generally closed cylindrical containers equipped with mechanical homogenization systems. These are particularly suited for industrial lactic fermentation, as they enable simultaneous feeding and extraction. Their high efficiency optimizes yields, ensuring a sustained production of bioproducts.
In continuous bioreactors, the substrate is consistently fed into the reactors while the product is simultaneously extracted. This creates a steady –state system where nutrients and microorganisms stay stable over time.
Key advantages include:
- Large scale production: high demand benefits from their efficiency, avoiding pauses for cleaning and restarting cycles.
- Consistency: they can run uninterrupted for extended periods.
- Accelerate microorganism growth: nutrients are consumed rapidly, requiring a balanced supply for best performance.
- Cost optimization: reduced downtime results in savings on raw materials and operational costs.
These bioreactors are particularly recommended for high-volume production due to their outstanding performance in full-scale operations. Proper maintenance is crucial to prevent cross contamination and ensure seamless operation.
Added benefits:
- No interruptions: substrates are incontiguous use unless a shutdown is needed.
- Increased productivity: higher product quantities are achieved in shorter periods.
Continuous bioreactors are specially used in industrial biotechnology as biofuel production, such as biodiesel, which requires a constant supply to meet high demand. They are also extremely used in the food and beverage industries, where fermentation is essential for producing enzymes and amino acids.
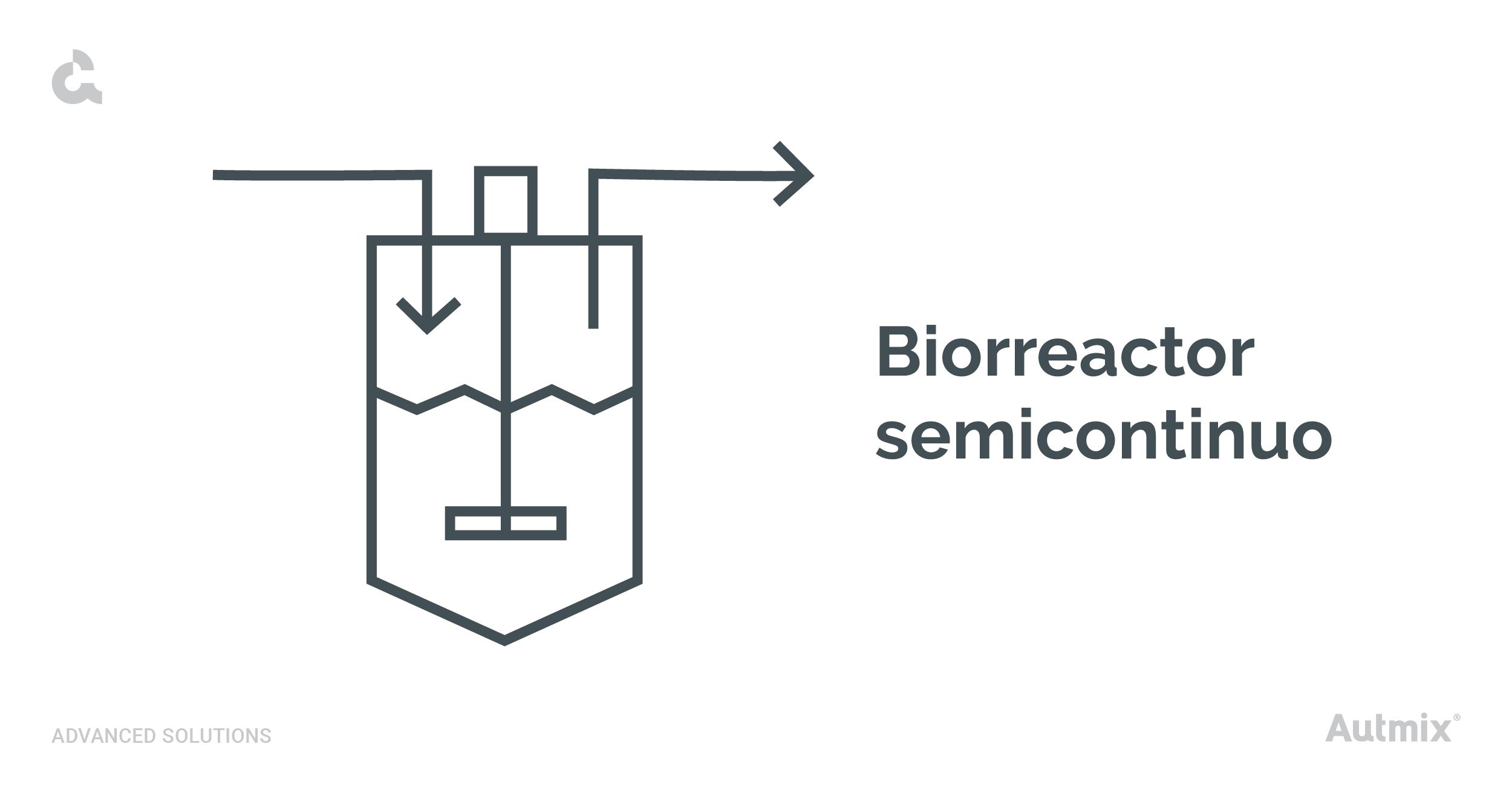
Semicontinuous bioreactors
Also known as fed-batch bioreactors, these systems combine features of both batch and continuous processes. They begin as batch systems but are gradually supplied with nutrients in a controlled manner without interruption.
Recommended application for semicontinuous bioreactors includes:
- Specific products: form antibiotics, proteins, or enzymes, controlled feeding perfects the growth phase.
- Inhibitory substrates: when the substrates inhibit growth, the semicontinuous system allows gradual dosing.
- Cell production: offers precise control of the growth phase, increasing cell density and biomass production without requiring stoppages.
- Flexible operations: the combination of batch and continuous characteristics ensures a consistent nutrient supply.
Implementing semicontinuous bioreactors offers several industrial advantages, particularly for antibiotics, proteins, food, beverage, and cell cultures.
Another classification: bio reactors by functioning
In industrial application, stirred-tank bioreactors are commonly recommended. These are characterized by a shaft with impellers designed to integrate and homogenize the product, keeping the best environment for elements such as yeast, cells, or bacteria.
Their construction is tailored to industrial scaling criteria, set up by engineering teams responsible for transitioning the bioprocess from laboratory to production.
Industrial scaling bioreactors
Bioreactors play a vital role in the production of diverse products essential to various industries. However, the scale up of bioreactors is a complex process, as it involves creating the necessary conditions for cell development.
For producers, the scale up process of bioreactors in several industries ensures not only product quality but also throughput and efficiency.
Advantages of scaling include:
- Large batches: increased yield by handling larger production volumes.
- Meeting high demand: producing more in less time is ideal for companies with significant demand.
For applications like lactic acid bacteria, semicontinuous bioreactors are selected due to their:
- Precise control of fermentation time allows best management of each phase through monitoring and adjustment.
- Adaptability: the batch system can be customized from the first stage to the end of fermentation without requiring continuous feeding.
Industrial fermentation products include:
In industrial scales, fermentation must be customized to balance quality and efficiency. Laboratory and pilot trials ensure that increased volumes do not compromise factors like mass transfer and yield.
When designing a scaled-up bioreactor, factors like height-to-diameter ratio must be considered. Calculation and simulation tools help ensure proper choice of materials, agitators, and performance while keeping consistent conditions.
Advantage of scaling up with an experienced company include:
- Consistency in production.
- Cost-effectiveness.
- Flexibility in managing large volumes without compromising conditions in industrial plants.
- Reduced operating costs: controlled operations minimize waste and improve the use of ingredients, water, oxygen, and electricity.
Industrial bioreactors can incorporate CIP (Cleaning-in-Place) systems, reducing maintenance time for cleaning, positively affecting labor use and improving productivity.
By adding scientific efforts with equipment for the biotechnology sector and the experience of highly trained allies, such as Sartorius and Autmix, the scaling of bioreactors at an industrial level becomes a strategic and sustainable investment.
See how we can help you optimize your current projects. We have a tailored solution.
Reduce your operating costs
I want to be contacted